1
Processing can be broken into three main parts. These parts overlap and may need to happen simultaneously during processing, so it is helpful to start on each part and plan tasks accordingly.
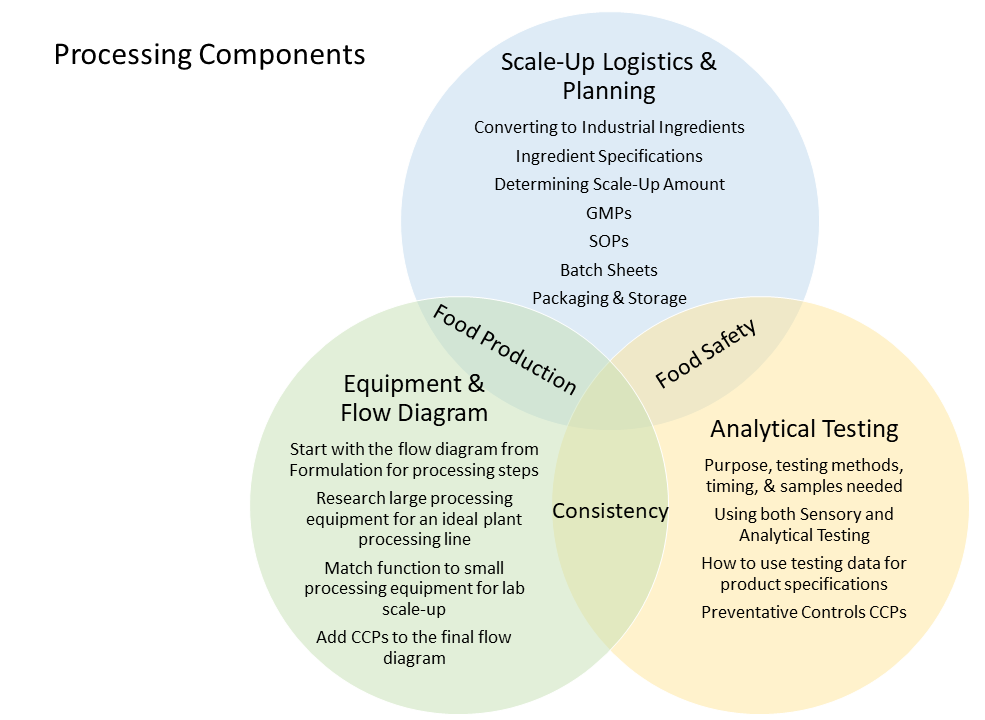
At the end of Processing, you will have completed the following components:
- Formulations – Gold Standard and Scale-up Formulation with Industrial Ingredients
- Analytical Testing Methods used, the purpose of test, method, and results
- Comparison of (Analytical and Sensory) Test Data from Scale-up to your Gold Standard
- Scale-Up Amount Calculated & Justified
- Equipment Needed for Large Scale Production – equipment photos and functionality
- Equipment Needed for Small Scale Lab Production – equipment settings and functionality
- GMPs, SOPs, and Completed Batch Sheets
- Ingredient Specifications
- Preventative Control Hazard Analysis and Flow Diagram with Preventative Controls Included
- Scale-up Summary – How did it go, successes, problems, etc.
Details of many of the components are included below as a reference. Keep in mind that you may not work in the order listed. This is meant to give you a scope of the tasks in an order that could likely be used.
Step 1. Gold Standard – Collect Analytical Data
- What are the most important characteristics of your product?
- Analytical Equipment typically available in-house includes:
- Water Activity Meter
- Moisture Balance
- pH Meter
- Refractometer
- Hunter Colorimeter
- Texture Analyzer – know what textural attribute you want to measure
- Bostwick Consistometer
- Ring Spread Test
- Brookfield Viscometer
- Penetrometer
- Seed Displacement
- Density (calculation)
Step 2. Convert to Industrial Ingredients
- Going away from fresh ingredients like milk, eggs, and raw fruits & vegetables
- If a fresh ingredient is key to your product, you will need to collect data to show that importance.
- Work on your industrial ingredient list due at the end of formulation/beginning of processing.
- The process of ordering industrial ingredients takes time, so try to be patient.
- See Industrial Ingredients Chapter and Worksheet in Formulation for more information.
Step 3. Production Equipment, Scale-Up Equipment, and Flow Diagram
- This covers a large segment of processing and includes multiple components.
- Production Equipment – equipment you would use in large-scale plant production – search references and the internet for processing equipment photos and videos. If you cannot find exactly what you are looking for, show a similar piece of equipment and explain how it would be modified for your product or sketch the new piece of equipment.
- Scale-Up Equipment – what you will use for scaling up your product on campus. The goal is to match the function of the large-scale equipment chosen with the scale-up equipment that is available.
- Flow Diagram – steps of your process written out in block diagram format. Take your initial flow diagram from Formulation and convert it for large-scale / plant production equipment and processing steps.
Step 4. Prepare GMPs, SOPs, & Batch Sheets
- GMPs: Read the cGMPs in 21 CFR Part 117. Then write your product-specific GMPs.
- SOPs: Standard Operating Procedures are detailed instructions for each step of your process. Think about handing the SOPs to a plant worker. Would they be able to make your product to your standards based on the instructions laid out in the SOPs?
- Batch Sheets: documents used in plants to monitor processes to ensure that the process is being carried out correctly and as a historical document that can be reviewed if there are any questions about the process in the future.
- For each step in your process (flow diagram), there should be a section in your batch sheet to document the step.
Step 5. Ingredient Specifications – can think about this in terms of what ingredient (quality/characteristics) you would (and would not) accept into a plant to make your product.
- Industrial Ingredient Specifications can be used.
- If you are using a grocery store ingredient:
- Find a comparable industrial ingredient specification sheet
- Or – use the Nutrition Facts Panel and/or the USDA FoodData Central to create your own ingredient specification.
- Use references to set microbial specifications.
Step 6. Conduct a Hazard Analysis using Preventative Controls Methodology
- Conduct a Hazard Analysis using the Preventative Controls for Human Food manual formatting.
- Assume strong prerequisite programs and that the plant is specific to your product.
- Focus on pathogens, allergens, and metal fragments.
- Determine Preventative Controls for your process and product. Also, determine what other concerns can be managed through Supplier Programs and/or Prerequisite Programs.
- Discuss the hazard analysis and Preventative Controls with faculty
- Add the Preventative Controls to the flow diagram.
Step 7. Packaging & Storage
- Think about your product characteristics.
- What functionality does your product packaging need to have to protect your product and have a positive impact on shelf life?
- Research packaging types to choose the ideal packaging material(s) for your product. More information about packaging will be included in the Commercialization section, but it does not hurt to start researching packaging materials as you have time.
- Discuss packaging needs for the scaled-up product (may not be the same as your ideal packaging).
- Need refrigerator or freezer space for the scaled-up product? Check with faculty to coordinate space.
Step 8. Scale Up
- The actual scale-up will take one to two lab periods. During this time, your team will make enough product for testing needs.
- It is important to have all of the details and procedure parameters figured out before scale-up occurs because typically there are only enough time and resources to scale up once.
Step 9. Analytical Measurements
- Your main goals are to:
- Compare your gold-standard product to your scaled-up product
- Collect data related to shelf-life determination (pH, water activity, color, etc.)
- Collect data – in-process and finished product – to provide guidance for process and finished product specifications.
- Refer to Step 1 for the analytical equipment list.

