9 Nutrition and Metabolism: Diabetes and Antidiabetics
Nutrition and metabolism are foundational aspects of human health, influencing how the body uses nutrients to maintain energy, repair tissues, and regulate physiological functions. Central to these processes is the pancreas, a critical organ in glucose metabolism that plays a vital role in maintaining blood sugar balance through the secretion of two key hormones: insulin and glucagon. Insulin, produced by the beta cells of the pancreas, lowers blood glucose levels by facilitating the uptake of glucose into cells for energy. Conversely, glucagon, secreted by the alpha cells, raises blood glucose by stimulating the liver to release stored glucose. Together, these hormones work through a feedback mechanism to maintain glucose homeostasis, ensuring the body’s cells receive adequate energy while preventing damage caused by high or low blood sugar levels.
In diabetes, this delicate balance is disrupted. Type 1 diabetes occurs when the pancreas produces little to no insulin due to the autoimmune destruction of the beta cells. In type 2 diabetes, insulin production may be insufficient, or cells become resistant to insulin’s effects. As a result, both conditions lead to hyperglycemia or elevated blood sugar levels, which can cause long-term complications if left unmanaged. This chapter explores the critical roles of nutrition, the pancreas, and hormonal regulation in glucose metabolism, focusing on how disruptions in these systems lead to diabetes, hypoglycemia, and hyperglycemia and how these conditions are managed clinically.
As you progress through this chapter, you will gain an understanding of the distinct differences between diabetes mellitus type 1 and type 2, their underlying causes, and the clinical signs and symptoms that help distinguish them. You will also explore the physiology behind hypoglycemia and hyperglycemia, their respective treatments, and how these conditions impact overall patient care. We will delve into insulin therapy and oral anti-diabetic medications, examining their mechanisms of action, therapeutic indications, adverse effects, and nurses’ critical role in managing these treatments effectively.
Learning Objectives
- Differentiate between diabetes mellitus type 1 and type 2.
- Understand the etiology, pathophysiology, and clinical manifestations of hypoglycemia and hyperglycemia and their related treatments.
- Discuss insulin therapy in terms of mechanism of action, indications for use, adverse effects, and implications for the nursing process.
- Explain oral anti-diabetic medications in terms of mechanisms of action, indications for use, adverse effects, and implications for the nursing process.
I. The Endocrine System Basics
Diabetes is an endocrine disease. Understanding the endocrine system is necessary to understand why diabetes occurs.
The nervous system uses neurotransmitters to communicate, while the endocrine system uses hormones for chemical signaling. These hormone signals are sent by the endocrine organs. Hormones are transported primarily via the bloodstream throughout the body, where they bind to receptors on target cells, inducing a characteristic response. The glands in the endocrine system include the pituitary, thyroid, parathyroid, adrenal, pineal glands, adrenal glands, pancreas, uterus, ovaries, and testes.
An illustration of the endocrine system.
The endocrine system’s main goal is homeostasis, which is achieved by regulating hormone levels in the blood. If hormone levels are unregulated or abnormal, health problems like diabetes, high blood pressure, changes in mood and behavior, and weight gain can occur (Betts et al., 2022).
Some of these glands have both endocrine and nonendocrine functions. For example, the pancreas contains cells that function in digestion and secrete the hormones insulin and glucagon, which regulate blood glucose levels.
How Hormones Work
Hormones cause changes in target cells by binding to specific cell-surface or intracellular hormone receptors, molecules embedded in the cell membrane, or floating in the cytoplasm with a binding site that matches a binding site on the hormone molecule. In this way, even though hormones circulate throughout the body and come into contact with many different cell types, they only affect cells with the necessary receptors. Receptors for a specific hormone may be found on or in many different cells or limited to a small number of specialized cells. For example, thyroid hormones act on many different tissue types, stimulating metabolic activity throughout the body. Cells can have many receptors for the same hormone but often also possess receptors for different types of hormones. The number of receptors that respond to a hormone determines the cell’s sensitivity to that hormone and the resulting cellular response.
Additionally, the number of receptors available to respond to a hormone can change over time, increasing or decreasing cell sensitivity. In up-regulation, the number of receptors increases in response to rising hormone levels, making the cell more sensitive to the hormone and allowing for more cellular activity. Cellular activity is reduced when the number of receptors decreases in response to rising hormone levels, which is called down-regulation.
Hormones Associated with regulating blood glucose and their effects
| Endocrine glands related to Diabetes | Hormone | Effects |
| Adrenal (cortex) | Cortisol | Increases blood sugar levels |
| Pancreas | Insulin | Reduces blood glucose levels |
| Pancreas | Glucagon | Increases blood glucose levels |
Nutrition plays a vital role in maintaining homeostasis and influencing hormonal function. Overall, a balanced diet with limited ultra-processed food, a variety of fruits and vegetables, whole grains, and lean protein sources is important for endocrine health. This diet helps individuals maintain a healthy weight and decrease the risk of hormonal imbalances. The table below lists the glands that are key in secreting and regulating hormones and related nutritional factors.
Endocrine Glands and Their Role in Regulating Hormones
| Gland | Role | Related Nutritional Factors |
| Adrenal glands | Release cortisol (steroid hormone), commonly called the “stress hormone.” | Adequate protein intake (5–7 oz eq/day for adults) as part of a balanced diet |
| Hypothalamus | Instructs the pituitary gland when to release hormones | A diet low in ultra-processed foods, which decreases inflammation |
| Ovaries | Produces sex hormones, including estrogen and progesterone | Balanced diet with limited red meat and poultry |
| Pancreas | Controls the release of insulin and glucagon, which are involved in regulating blood glucose levels and affecting digestion | Limited added sugar, refined grains, and overall calories |
| Pineal gland | Produces melatonin, which helps regulate sleep | Foods containing melatonin have not been found to affect pineal gland function. |
| Pituitary gland | Known as the “master gland,” it influences many other endocrine glands, including the thyroid. It affects various bodily functions such as blood pressure, metabolism, and growth. | (< 2300 mg/day); a diet low in ultra-processed foods, which decreases inflammation |
| Testes | Produces the sex hormone testosterone | Foods or supplements containing omega-3 fatty acids |
| Thyroid | Controls metabolism, heart and digestive function, mood, and muscle and bone development | Adequate iodine intake through iodized salt, fish, shellfish, dairy, seaweed |
The focus will be on the pancreas. The pancreas contains “Beta cells are the producers of the only blood glucose-lowering hormone in the body: insulin. Alpha cells, by contrast, produce glucagon, a hormone that has blood glucose-increasing effects” (Moede, Leibiger, & Berggren (2020).
II. Review of Nutrition
Several food-related concepts are fundamental to understanding how nutrition affects the body. Food refers to edible substances made of protein, carbohydrates, fat, or other nutrients. Food is comprised of macronutrients and/or micronutrients. Macronutrients are water and energy-yielding nutrients (carbohydrates, fats, and proteins) needed in large amounts by the body. Micronutrients include vitamins and minerals. Food is the building block of the diet, which describes the quantity and quality of food and drinks consumed.
Recommended normal daily food breakdown: Macronutrients Energy Yield and Recommended Daily Intake (source: USDA & USHHS, 2020)
| Macronutrient | Calories per Gram | Recommended Daily Intake |
| Carbohydrate | 4 | 45–65% |
| Fat | 9 | 20–35% |
| Protein | 4 | 10–35% |
A thorough analysis of the client’s nutrition status can help identify potential endocrine-related issues. The nurse should obtain a nutritional assessment to identify any nutrient-related deficiencies. For example, a patient with limited fruit and vegetable consumption is at risk for vitamin C deficiency. Vitamin C deficiency is associated with multiple health problems. Specifically related to the endocrine system, vitamin C affects the regulation of several important hormones, including insulin growth factor and sex steroids (Shi, Rath, & Niedzwiecki, 2021).
Conversely, when evaluating the patient’s nutrition status, there is potential to find excessive intake of nutrients. For example, a diet with excessive carbohydrate intake can increase blood glucose levels in individuals, contributing to the development of insulin resistance and diabetes. Once nutritional imbalances are identified, the nurse can work with the patient to make dietary changes and reduce the risk of developing endocrine disorders (Bickley et al., 2021).
III. What is Diabetes Mellitus?
What is Diabetes?

The most prevalent endocrine disorder is diabetes (Agency for Healthcare Research and Quality, n.d.). It is estimated that 37.3 million Americans have diabetes, with approximately 8.5 million cases (23%) undiagnosed (National Institute of Diabetes and Digestive and Kidney Diseases, 2023). Dysfunction of insulin production and secretion and the target cells’ responsiveness to insulin can lead to diabetes mellitus. This common disease affects the ability of the body to produce and/or utilize insulin. There are two main forms of diabetes mellitus. Type 1 diabetes is an autoimmune disease affecting the beta cells of the pancreas. The beta cells of people with type 1 diabetes do not produce insulin; thus, synthetic insulin must be administered by injection or infusion. Type 2 diabetes accounts for approximately 95 percent of all cases. It is acquired, and lifestyle factors such as poor diet and inactivity greatly increase a person’s risk. In type 2 diabetes, the body’s cells become resistant to the effects of insulin. In response, the pancreas increases its insulin secretion, but the beta cells become exhausted over time. In many cases, type 2 diabetes can be reversed by moderate weight loss, regular physical activity, and consumption of a healthy diet. However, if blood glucose levels cannot be controlled, oral diabetic medication is implemented, and eventually, the type 2 diabetic may require insulin.
Risk Factors for Diabetes Mellitus:
Diabetes Mellitus Type 1:
- Body Mass Index greater than 25
- Caused by an autoimmune reaction (the body attacks itself by mistake)
- Family history: Risk increases if a parent or sibling has the disease
- Age: Any age but most prominent in children, teens, and young adults
- Race: most commonly found in Caucasians. and Latinos in the US
- Events that potentiate Diabetes Mellitus Type 1: Exposure to viral infection, e.g., Mumps; Trauma, e.g., car accident; Environmental factors, e.g., Climate
- Disease/disorders that potentiate Diabetes Mellitus Type 1: HIV, Pre-diabetes, Gestational diabetes
Diabetes Mellitus Type 2:
- Body Mass Index greater than 25
- Family history. Risk increases if a parent or a sibling has the disease
- Age: Individuals 45 years or older
- Race: seen most often in African American, Hispanic or Latino, American Indian, or Alaska Native persons. Some Pacific Islander people and Asian Americans
- Other factors that can potentiate Diabetes Mellitus Type 2: Obesity and Sedentary lifestyle
- Disease or disorders that potentiate Type 2 Diabetes: Non-alcoholic fatty liver disease (NAFLD), Gestational diabetes (diabetes during pregnancy), or giving birth to a baby who weighs over 9 pounds.
Signs and Symptoms of Diabetes:
Diabetes Mellitus Type 1:
Polyuria, Polydipsia, Polyphagia (called the 3 Ps), unexplained weight loss, blurred vision, extreme fatigue, slow-healing sores or infections, neuropathy, and possible gastroparesis
Diabetes Mellitus Type 2:
Polyuria, Polydipsia, Polyphagia (called the 3 Ps), unexplained weight loss, blurred vision, extreme fatigue, slow-healing sores or infections, neuropathy, and possible gastroparesis, areas of darkness under the armpits and neck

What causes Type 2 Diabetes:
Diabetes is diagnosed when lab tests reveal that blood glucose levels are higher than normal, a condition called hyperglycemia.
The most common laboratory tests used to diagnose Diabetes:

Tests explained:
A1C test. This blood test requires that the patient not eat (NPO) for a period of time (fasting) prior to the blood test or, more recently, a probe reading is obtained. This test demonstrates the patient’s average blood sugar level over the last 2 to 3 months.
It measures the percentage of blood sugar attached to hemoglobin, the oxygen-carrying protein in red blood cells. It’s also called a glycated hemoglobin test. An A1C between 5.7% and 6.4% means that you have prediabetes. Below 5.7% is considered normal.
Random blood sugar test. A blood sample is most often obtained from blood work or a sample obtained from a fingerstick taken at a random time. No matter when you last ate, a blood sugar level of 200 milligrams per deciliter (mg/dL)— or higher suggests diabetes.
Fasting blood sugar test. A blood sample is obtained after the patient has fasted from midnight the night before. A fasting blood sugar level of less than 100 mg/dL is normal. A fasting blood sugar level from 100 to 125 mg/dL is considered prediabetes. If it’s 126 mg/dL or higher on two separate tests, you have diabetes.
Glucose tolerance test. For this test, the patient will fast overnight. Then, the fasting blood sugar level is measured. Then the patient will drink a sugary liquid, and blood sugar levels are tested regularly for the next two hours.
A blood sugar level of less than 140 mg/dL is normal. A reading of more than 200 mg/dL after two hours means you have diabetes. A reading between 140 and 199 mg/dL means you have prediabetes.
IV. Nutrition and the Plan of Care for the Diabetic Patient
Patients with diabetes should follow the prescribed diet, which typically includes a limited number of calories and carbohydrates. General guidelines for meal planning with diabetes include (CDC, 2023b):
- Including more non-starchy vegetables
- Limiting added sugars and refined grains such as white bread, rice, and pasta
- Restricting intake of highly processed foods
- Limiting high-fat meat, full-fat dairy products, sweeteners, ultra-processed foods, and trans fats.
- Note: Excessive added sugar, refined grains, or overall calories can cause or worsen this condition.

The Plan of Care for the Patient with Diabetes:
Endocrine disorders can be difficult to diagnose. Frequently, these conditions develop over time, and patients present with non-specific symptoms. A thorough history and physical are essential.
Health History and Physical Assessment
Taking a patient’s health history is the first step when assessing the patient’s endocrine system. In addition to general health history questions, it is important to identify conditions for which the patient may be at risk and to assess the need for genetic testing (Bickley et al., 2021). When collecting information on the patient’s nutritional status for potential effects on the endocrine system, the nurse should ask about changes in appetite, thirst and fluid intake, and bowel function. It is also important to know if the patient has experienced any changes in weight or energy levels.
After obtaining a thorough health history, a physical assessment should be performed to observe for signs of endocrine dysfunction:
- Inspect the body, looking for abnormal hair growth, skin changes, and abnormal body proportions.
- Auscultate heart sounds for rate and rhythm, as some endocrine disorders cause tachydysrhythmias. Also, listen over the carotid arteries and directly over the thyroid gland for any bruits (here is a link to listen to a bruit: https://www.youtube.com/shorts/ToL3vuvdZA0?feature=share)
- Palpate the thyroid gland, adrenal glands, and testes for tenderness, size, symmetry, and shape, looking for any nodules, enlargement, or changes in texture.
- Assess weight, height, and body mass index (BMI) for any changes in body composition.
- Assess sexual development, including secondary sex characteristics and menstrual cycles.
- Evaluate the patient’s mood and behavior for any changes.
- Perform point-of-care blood glucose testing (Bickley et al., 2021).
Other assessment needs:
- Assess education level to determine the best educational tools that can be used to teach the patient about diabetes
- Determine the patient’s ability to access/obtain healthy foods and medication, e.g., finances, transportation, mobility
Patient’s nutritional goals:
It is important to set nutritional goals with individuals with endocrine disorders, as diet is crucial in managing these conditions and promoting overall health and well-being. Research has shown that dietary modifications can significantly improve outcomes and reduce the risk of complications associated with various endocrine disorders, particularly diabetes. For example, research has shown that dietary interventions, including reducing simple carbohydrate and added sugar intake while increasing protein and fiber intake, can improve blood glucose control and reduce the risk for cardiovascular disease in individuals with type 2 diabetes (Gray & Threkeld, 2019).
The American Diabetes Association (2019) has identified a framework for nutrition therapy goals that reflect the importance of using a holistic approach to working with patients with diabetes. Their broad goals of nutrition therapy are to:
- Promote and support healthful eating patterns, emphasizing a variety of appropriately portioned nutrient-dense foods.
- Consider personal and cultural preferences, health literacy, willingness to change, and access to healthy foods.
- Do not judge patients about their food choices so that their eating can continue to be enjoyable.
- Provide practical tools for developing good eating practices rather than focusing too much on a particular food item or food type.
Restaurants’ portion sizes have increased over time, making it difficult for patients to accurately measure food servings and follow their prescribed diet. This video above demonstrates how to measure portion sizes using your hands—no measuring tools are required—and is a resource.
Nursing Interventions:
The nurse should cover the following items as part of monitoring carbohydrate intake for clients with diabetes (CDC, 2023a):
- Patients with diabetes should track their carbohydrate intake to better manage their blood glucose levels
- Keeping blood glucose levels stable and as close to normal as possible will help the patient feel well and prevent or delay the development of diabetes-related complications.
- There are three types of carbohydrates:
- Sugars: naturally found in fruit or milk or added to many foods (added sugars are identified on the food label)
- Starches: grains and starchy vegetables such as corn or potatoes
- Fiber: indigestible part of plants that do not elevate blood glucose levels
- Carbohydrates are measured in grams, listed on food labels, or found in resources listing nutritional information for nonpackaged food items.
- One serving of carbohydrates = 15 g of carbohydrates.
- The patient should follow their prescribed diet indicating how many servings of carbohydrates to consume (here is a website that teaches how to count carbohydrates: https://diabetes.org/food-nutrition/understanding-carbs/carb-counting-and-diabetes)
- The patient should try to consume consistent carbohydrates at each meal to maintain consistent blood glucose levels.
Evaluation Plan:
- The plan’s effectiveness is evaluated by measuring expected clinical outcomes.
- Subjective data: the patient reports an improvement in their symptoms.
- Objective measures can also be evaluated: Lab tests, including finger sticks and HbA1c, indicate glycemic improvement or control.
If the goals were not met, the plan needs to be adjusted. Perhaps the plan was too difficult for the patient to follow and involved too many dietary changes all at once. The updated plan could initially focus on prioritizing and managing fewer nutrients and then expand as the patient can manage the changes. If the patient follows the plan but does not see the expected outcomes, work with the health care provider and dietician to adjust the plan.
Unfolding Case Study for Diabetes Mellitus:
Part A
Justin Owens (JO) is a 25-year-old Hispanic male who is coming in to see his Primary Care Provider for frequent thirst, hunger, and urination, with an unexplained 10 lb. weight loss in the last 6 weeks.
History and Physical (H & P)
History (subjective data)
Allergies: Penicillin leads to anaphylaxis, and seafood leads to all over rash and swelling of lips, denies any environmental allergies.
PMH: Dust mite allergy, several episodes of bronchitis as a child, recently early sense of fullness and heartburn
PSH: ORIF to left tibia from a fracture that occurred while playing soccer
FH: Mother has Hypertension (HTN), Diabetes Mellitus type 1, and Hypercholesterolemia; Father and all paternal relatives have been diagnosed with Diabetes Mellitus (DM) type 2 and Acid Reflux
Activity: Runs two miles every morning
Social History:
Drinks two to three beers with friends on the weekends or when watching soccer on TV. He smoked marijuana for 6 months during his senior year in high school. No other illicit drugs were taken. Has never smoked cigarettes.
Medications: Claritin 10 mg po q am
Occupation: Army Corp Engineer
Hobbies: Camping and cooking
Review of Systems: Patient complains of occasional blurred vision and mild right upper quadrant. Consistent polyuria, polydipsia, and polyphagia, unexplained weight loss, and fatigue,
Questions to test newly obtained knowledge from the OER:
- What risk factors does JO have for Diabetes Mellitus type 1?
- What tests would the healthcare team order at this time?
Teaching diabetic patients the option to self-monitor glucose levels
Unfolding Case Study Part B:
JO’s physical assessment starts with obtaining vital signs:
T 98°F, P 70 bpm, 18 bpm, 118/60 mmHg
The physical assessment findings:
He is alert and oriented x4 and comprehends all questions and statements well. Cranial nerves II-VII are grossly intact, but vision is blurred when the patient reads for long periods of time, and mild tingling and burning are noted to the toe and bottom of feet after long days of standing at work. Skin turgor is tented, and tongue is dry. Upper and lower extremity strength is 5/5 and movements are coordinated, active ROM noted to all four extremities. Skin intact, with slight edema noted in the ankles, but wounds now take longer to heal. S1 and S2 were noted without ectopy with regular rate and rhythm. Thorax is normal in shape and size, and lung sounds are clear throughout lung fields with regular rate, rhythm, and depth. The abdomen is soft, non-distended, and non-tender, with positive bowel sounds in all four quadrants. Bowel movements are noted every morning, and infrequent upper right quadrant pain is reported. Appetite, oral intake, and urination have increased, and the Fasting fingerstick is 260 mg/dl. Acid reflux has also been noted frequently.
The urine sample indicates clear yellow urine with an aromatic odor. Abnormal blood work results include:
- What data in the physical assessment findings indicates that JO has Diabetes Mellitus Type 1?
- What non-pharmacological interventions are recommended for the patient now?
V. Unstable Diabetes Mellitus
Hypoglycemia and Hyperglycemia:
The overlapping symptoms of hypo- and hyperglycemia (e.g., hunger, sweating, trembling, confusion, irritability, dizziness, blurred vision) make the two conditions difficult to distinguish from one another (Paradalis, 2005). Since the treatment is different for each condition, testing the patient’s blood glucose when symptoms occur is critical. The risk factors that may have led to the condition, as well as the patient’s recent medical history, also help to determine the cause of symptoms.
Hypoglycemia
Hypoglycemia occurs in diabetic patients with a blood glucose of less than 70 mg/dL. If glucose continues to remain low and is not rectified through treatment, a change in the patient’s mental status will result. Patients with hypoglycemia become confused and experience headaches. Left untreated, they will progress into semi-consciousness and unconsciousness, leading rapidly to brain damage. Seizures may also occur.
Common initial symptoms of hypoglycemia include:
- Cold, clammy skin
- Weakness, faintness, tremors
- Headache, irritability, dullness
- Hunger, nausea
- Tachycardia, palpitations
These symptoms will progress to mood or behavior changes, vision changes, slurred speech, and unsteady gait if the hypoglycemia is not properly managed.
The hospitalized patient with type 1 or type 2 diabetes is at an increased risk for developing hypoglycemia. Potential causes of hypoglycemia in a hospitalized diabetic patient include:
- Receiving insulin and some oral antidiabetic medications (e.g., glyburide)
- Fasting for tests and surgery
- Not following the prescribed diabetic diet
- New medications or dose adjustments
- Missed snacks
Hypoglycemia (a blood glucose level below 70 mg/dL) must be treated immediately to avoid serious complications. The 15-15 Rule can be used to treat mild hypoglycemia when blood glucose levels decrease below 70 mg/dL. Patients with blood glucose levels in this range should consume 15 g of carbohydrates and then recheck their blood glucose level after 15 minutes. Repeat these steps until their blood glucose is greater than 70 mg/dL. Examples of food items that have approximately 15 g of carbohydrates include:
- 4 oz of juice
- 1 tbsp of sugar, honey, or syrup
- Small portions of candy (hard candy, jellybeans, etc.)
Blood glucose levels that do not respond to these interventions require immediate attention at a healthcare facility (CDC, 2022b). An initial blood glucose reading may confirm suspicion of hypoglycemia. If you suspect your patient is hypoglycemic, obtain a blood glucose level through a skin puncture. A 15 g oral dose of glucose should be given to increase blood glucose by approximately 2.1 mmol/L in 20 minutes (Canadian Diabetes Association, 2013). See the outline below for an example of a protocol that may be used to treat hypoglycemia.
| Hypoglycemia Treatment Protocol-most used in hospital or inpatient treatment | |||
| Disclaimer: This is an example only of a hypoglycemia protocol. Always follow the protocol of your agency. | |||
| Capillary Blood Gas (CBG) | Able to Swallow | Nil per Mouth with IV Access | Nil per Mouth with No IV Access |
|
>72 mg/dl |
No treatment necessary | No treatment necessary | No treatment necessary |
|
40-70 mg/dl |
Give 15 g of glucose in the form of:
Note: Milk, orange juice, and glucose gels increase blood glucose (BG) levels more slowly and are not the best choice unless the above alternatives are unavailable. Repeat CBG every 15 to 20 minutes and repeat above if BG remains below 4 mmol/L. Once BG reaches 4 mmol/L/72mg/dl, give the patient 6 crackers and 2 tablespoons of peanut butter. If a meal is less than 30 minutes away, omit snacks and give the patient a meal when it is available. |
Notify physician.
Give 10-25 g (20-50 ml of D50W — dextrose 50% in water) of glucose intravenously over 1 to 3 minutes, OR as per agency policy. Repeat CBG every 15 to 20 minutes until 4 mmol/L. Continue with BG readings every 30 minutes for 2 hours.
|
Notify physician.
Give glucagon 1 mg subcutaneously (SC) or intramuscularly (IM). Position the patient on the side. Repeat CBG every 15 to 20 minutes. Give a second dose of glucagon 1 mg SC or IM if BG remains below 4 mmol/L. |
|
< 40 mg/dl |
Call lab for STAT BG level.
Continue as above. |
Call lab for STAT BG level.
Continue as above. |
Call lab for STAT BG level.
Continue as above. |
| Data source: Canadian Diabetes Association, 2013; Paradalis, 2005; Rowe et al., 2015; VCH 2009 |
One of the medications used to treat severe hypoglycemia:
Hyperglycemia
Hyperglycemia occurs when blood glucose values are greater than 126 mg/dl in a fasting state or greater than 180 mg/dl two hours after eating a meal (Pardalis, 2005). Hyperglycemia is a serious complication of diabetes that can result from eating too much food or simple sugar; insufficient insulin dosages; infection, illness, surgery, and emotional stress. Surgical patients are particularly at risk for developing hyperglycemia due to the surgical stress response (Dagogo-Jack & Alberti, 2002; Mertin, Sawatzky, Diehl-Jones, & Lee, 2007). Classic symptoms of hyperglycemia include the three Ps: polydipsia, polyuria, and polyphagia.
The common symptoms of hyperglycemia are:
- Increased urination/output (polyuria)
- Excessive thirst (polydipsia)
- Increased appetite (polyphagia), followed by lack of appetite
- Weakness, fatigue
- Headache
Other symptoms include glycosuria, nausea and vomiting, abdominal cramps, and progression to diabetic ketoacidosis (DKA).
Potential causes of hyperglycemia in a hospitalized patient include:
- Infection
- Stress
- Increased intake of calories (IV or diet)
- Decreased exercise
- New medications or dose adjustments
Testing blood glucose levels too soon after eating will result in higher blood glucose readings. Blood glucose levels should be taken one to two hours after eating.
If hyperglycemia is not treated, the patient is at risk for developing DKA. This is a life-threatening condition in which the body produces acids called ketones due to breaking down fat for energy. DKA occurs when insulin is extremely low, and blood sugar is extremely high.
VI. Complications of Diabetes Mellitus
DKA presents clinically with symptoms of hyperglycemia as above, Kussmaul respiration (deep, rapid, and labored breathing that is the result of the body attempting to blow off excess carbon dioxide to compensate for the metabolic acidosis), acetone-foul-odor breath, nausea, vomiting, and abdominal pain (Canadian Diabetes Association, 2013). Patients in DKA also undergo osmotic diuresis. They pass large amounts of urine because of the high solute concentration of the blood and the body’s attempts to get rid of excess sugar.
DKA is treated with the administration of fluids and electrolytes such as sodium, potassium, and chloride, as well as insulin. Be alert for vomiting and monitor cardiac rhythm. Untreated DKA can be fatal.

Patients with hyperglycemia may also exhibit a non-ketonic hyperosmolar state, known as hyperglycemic hyperosmolar syndrome (HHS). This is a serious diabetic emergency with a mortality rate of 10% to 50%. Hyperosmolarity occurs when the blood has a high sodium and glucose concentration, causing water to move out of the cells into the bloodstream.
Glycemic control, which is the maintenance of optimal blood glucose levels, is essential to manage potential complications of diabetes effectively. Poorly controlled blood glucose levels lead to vascular and neurological complications, including myocardial infarctions, cerebral vascular accidents, renal disease, neuropathies, and retinopathy (Sapra & Bhandari, 2023). These complications result from several different mechanisms triggered by elevated blood glucose levels that either cause buildup within vessels that impede blood flow, reduce the ability of the vessels to autoregulate, or trigger altered biochemical processes that hinder the vessel’s functionality (Brutsaert, 2022). The risk of developing these conditions is increased in patients with co-morbid conditions.
The plan of care for each diabetic patient must be multi-faceted. To help the patient achieve glycemic control, the following must occur:
- Risk assessment for diabetes
- Obtain an initial and, as needed, health history and physical assessment
- Get orders or use protocols to obtain serum glucose level, Hemoglobin A1C, especially if DM type 2 is suspected, another lab test, and a fingerstick
- Once diagnosed with Diabetes type 1 or type 2 teaching self-care is imperative, and the nurse will have to address the following:
- Assess for readiness to learn the diagnosis of Diabetes can be overwhelming. The patient may need time to digest this new information before being mentally and mindfully ready to learn about the changes that need to occur daily within their normal routine.
- Assess how the patient’s educational, emotional, social, and spiritual needs, socioeconomic conditions, cultural norms, and religious beliefs affect their nutritional intake and learning ability.
- If possible, ensure that teaching occurs in a family setting or with at least one support person present and is age-appropriate.
- Address changes to modifiable risk factors.
- Discuss the need for glucose monitoring and frequent medical follow-up, especially initially, after hospitalizations, or after frequent severe Hyperglycemic or Hypoglycemic events
- Nutritionist for needed diet changes and weight loss, especially with type 2 diabetes.
- Assess the patient’s accessibility to nutritious food.
- Identify and start an exercise program via a sport or exercise routine the patient likes.
- Starting/ giving self, oral, or subcutaneous medication as ordered
- Completing skincare especially foot care, and being meticulous with hygiene
- How to take medication and discuss possible medication side or adverse effects
- Medical follow-up as prescribed
Unfolding Case Study Part C:
The plan of care for JO was initiated using non-pharmacological measures. Six months later, he is 170 lbs. and his vital signs are: T: 98 °F, P: 77 bpm, R: 16 bpm, 114/70 mmHg, and a fasting fingerstick of 150 mg/dl
He has kept a food journal indicating that he is eating less fatty meat and less full fat dairy products and has added more fruits and vegetables to each meal. He continues to exercise everyday and to monitor his glucose level before meals and at bedtime but blood glucose levels are still above normal parameters.
An updated H & P:
History: The patient states that the polyuria and polydipsia have improved, but he is still eating more than usual. The abdominal pain and heartburn continue.
Physical: No changes noted
Questions:
- What teaching would JO need now that insulin will be part of the care plan??
- What other teaching concerning acid reflux and lower extremity care is needed?
- What teaching is needed to address hypoglycemia and hyperglycemia?
VII. Antidiabetics
Pancreatic Basics: A&P Review
Pancreas
The pancreas is a long, slender organ located near the stomach. Although it is primarily an exocrine gland, secreting a variety of digestive enzymes, the pancreas also has an endocrine function. Pancreatic islets, clusters of cells formerly known as the islets of Langerhans, secrete glucagon and insulin. Glucagon plays an important role in blood glucose regulation because low blood glucose levels stimulate its release. On the other hand, elevated blood glucose levels stimulate the release of insulin.
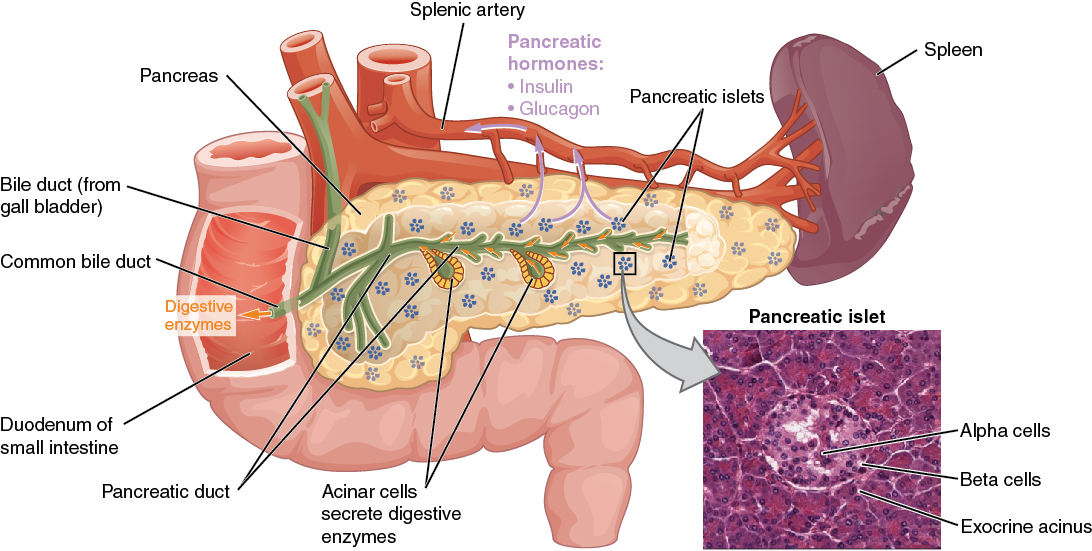
Regulation of Blood Glucose Levels by Insulin and Glucagon
Glucose is the preferred fuel for all body cells. The body derives glucose from the breakdown of the carbohydrate-containing foods and drinks we consume. Glucose not immediately taken up by cells for fuel can be stored by the liver and muscles as glycogen or converted to triglycerides and stored in the adipose tissue. Hormones regulate both the storage and the utilization of glucose as required. Receptors located in the pancreas sense blood glucose levels, and subsequently, the pancreatic cells secrete glucagon or insulin to maintain normal levels.
Glucagon
Receptors in the pancreas can sense the decline in blood glucose levels, such as during periods of fasting or during prolonged labor or exercise. In response, the alpha cells of the pancreas secrete the hormone glucagon, which has several effects:
- It stimulates the liver to convert stores of glycogen back into glucose. This response is known as glycogenolysis. The glucose is then released into the circulation for use by body cells.
- It stimulates the liver to take up amino acids from the blood and convert them into glucose. This response is known as gluconeogenesis.
- It stimulates lipolysis, the breakdown of stored triglycerides into free fatty acids and glycerol. Some of the free glycerol released into the bloodstream travels to the liver, which converts it into glucose. This is also a form of gluconeogenesis.
Taken together, these actions increase blood glucose levels. The activity of glucagon is regulated through a negative feedback mechanism; rising blood glucose levels inhibit further glucagon production and secretion.
Glucagon
Glucagon is indicated as a treatment for severe hypoglycemia (low blood sugar), which may occur in clients with diabetes mellitus. Glucagon injection is used for clients who are unable to safely swallow carbohydrates to treat hypoglycemia due to the effects of hypoglycemia or other medical conditions.
Mechanism of Action
Glucagon increases blood glucose concentration during an episode of hypoglycemia. See the image of an emergency glucagon kit.
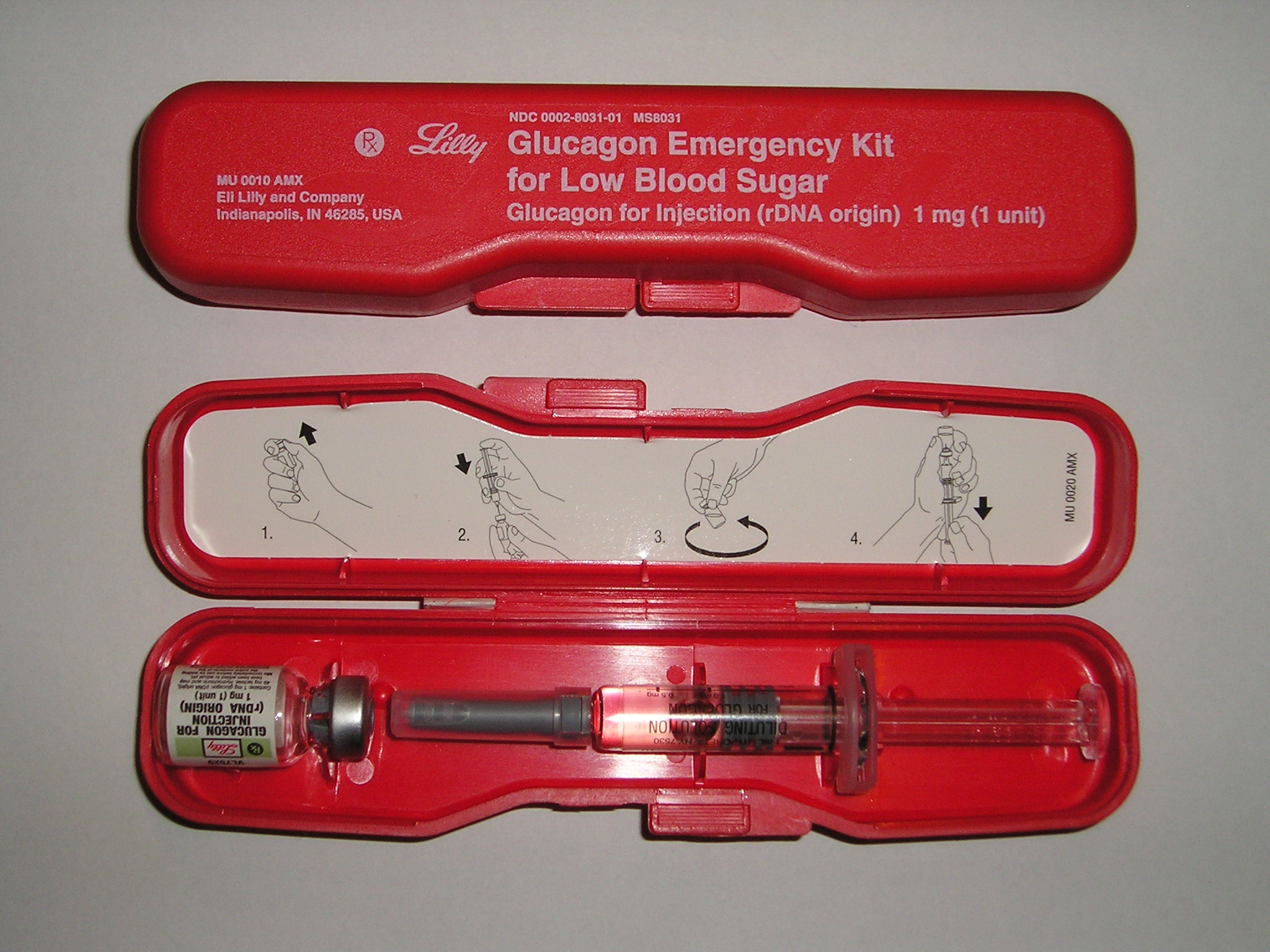
Specific Administration Considerations
Glucagon may be administered subcutaneously, intramuscularly, or intravenously. Peak glucose levels occur within 13-20 minutes of subcutaneous or IM injection.
Patient Teaching & Education
Patients with type 1 diabetes may have less of an increase in blood glucose levels compared with a stable type 2 client, so a supplementary carbohydrate should be given as soon as possible, especially to a pediatric client.
Illustration of homeostatic regulation of blood glucose levels
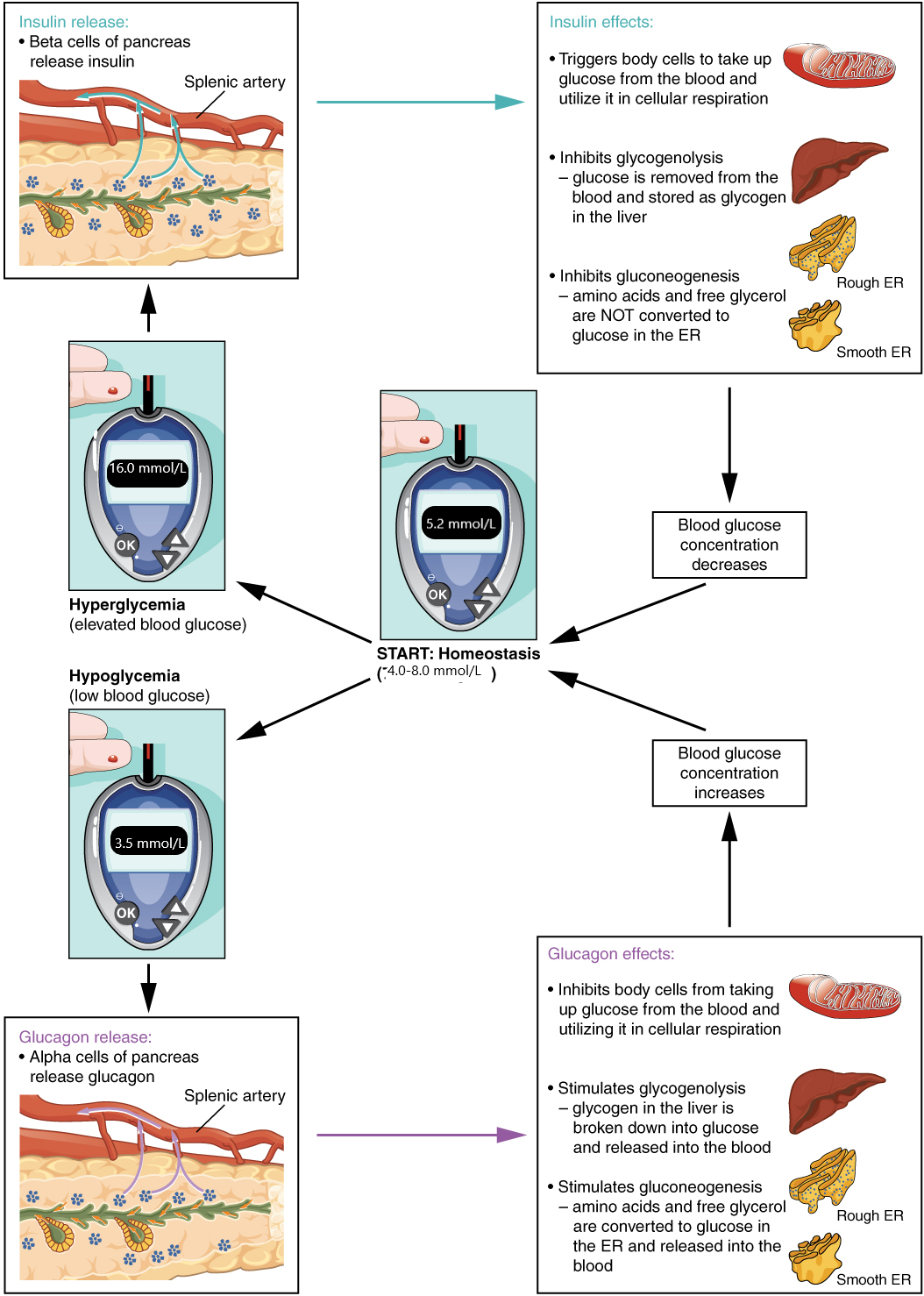
Insulin
Insulin facilitates the uptake of glucose into skeletal and adipose body cells. The presence of food in the intestine triggers the release of gastrointestinal tract hormones. This, in turn, triggers insulin production and secretion by the beta cells of the pancreas. Once nutrient absorption occurs, the resulting surge in blood glucose levels further stimulates insulin secretion.
Insulin triggers the rapid movement of glucose transporter vesicles to the cell membrane, where they are exposed to the extracellular fluid. The transporters then move glucose by facilitated diffusion into the cell interior.
Insulin also reduces blood glucose levels by stimulating glycolysis, the metabolism of glucose for generation of ATP. It further stimulates the liver to convert excess glucose into glycogen for storage, and it inhibits enzymes involved in glycogenolysis and gluconeogenesis. Finally, insulin promotes triglyceride and protein synthesis. The secretion of insulin is regulated through a negative feedback mechanism. As blood glucose levels decrease, further insulin release is inhibited.
Basal Insulin can be long-acting (insulin glargine or insulin detemir) or intermediate-acting (insulin isophane suspension [NPH]). Prandial insulins are used with meals and may be rapid acting (insulin lispro, insulin aspart, or insulin glulisine) or short acting (regular insulin).
Insulin requirements can be estimated based on weight, with typical doses ranging from 0.4 to 1.0 units/kg/day. Higher amounts are required during puberty, pregnancy, and medical illness. Physiologic insulin secretion varies with glycemia, meal size, and tissue demands for glucose. Strategies have evolved to adjust meal-time doses based on predicted needs to approach this variability in people using insulin treatment. Patient education on how to adjust insulin to account for carbohydrate intake, premeal glucose levels, and anticipated activity is important. Ensuring that patients and/or caregivers understand the correct insulin injection technique is also important to optimize glucose control and insulin use safety.
Patients on insulin therapy are at risk for hypoglycemia. It is essential for the nurse to monitor for signs of hypoglycemia and to intervene appropriately.
Hypoglycemia Symptoms to monitor when a patient is on insulin therapy:
| Mild-to-Moderate | Severe |
|
|
If a patient with diabetes shows a sudden change in mood or mental status or other symptoms of hypoglycemia, the nurse should immediately check the blood glucose level. Healthcare agencies use hypoglycemia protocols so that the nurse can react quickly to episodes of hypoglycemia before they become severe. Hypoglycemia protocols contain orders for immediate treatment by the nurse. For instance, in patients who can tolerate oral intake, 15-20 grams of rapidly digested carbohydrates (such as 250 mL of fruit juice) are recommended. In patients who are NPO or can’t take oral treatment, dextrose 50% IV or glucagon IM or subcutaneously are administered. Patients who have had a hypoglycemic episode should be monitored closely for the following 24 hours because they are at increased risk for another episode. The provider and the oncoming nurse should be notified of hypoglycemia episodes to discuss the possible cause of the hypoglycemic event and make insulin adjustments, if needed, to avoid additional hypoglycemia. Tracking hypoglycemia episodes and analyzing causes are important performance improvement activities.
The arrival of continuous glucose monitors to clinical practice has been proven to reduce nocturnal hypoglycemia in people using insulin pumps with glucose sensors due to automatic suspension of insulin delivery at a preset glucose level. A hybrid closed-loop pump system automatically adjusts basal insulin delivery every 5 minutes based on sensor glucose to maintain blood glucose levels as close to a specific target as possible.
According to the ADA, lifestyle modifications that improve health should be emphasized along with any pharmacologic therapy. Lifestyle modifications include healthy food choices to stabilize blood glucose levels and daily exercise.
Hypokalemia
All insulin products cause a shift in potassium from the extracellular to intracellular space, which can possibly lead to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients taking insulin and those at risk for hypokalemia due to other medications such as diuretics.
VIII. Anti-diabetic Medication: Subcutaneous and Oral Medications
Insulin Pens
Insulin pens are often used in inpatient settings, as well as for self-administration, to facilitate safe and accurate self-administration of insulin. See the image below of an insulin pen. According to the ISMP, insulin pens offer several advantages over vials beyond dosing accuracy, convenience, and ease of use:
- Each pen is already labeled by the manufacturer with the product name and product barcode (whereas syringes of insulin prepared on the patient care unit from vials run the risk of being unlabeled).
- Each pen can be individually labeled with the patient’s name (and ideally with a patient-specific barcode).
- The pen provides the patient’s insulin in a form ready for administration.
- The pen lessens nursing time needed to prepare and administer insulin.
- Insulin pens reduce medication waste that can occur when dispensing 10 mL-sized insulin vials for each client.
However, improper sharing of insulin pens among multiple patients has exposed patients to bloodborne pathogens. Insulin pens should never be reused for multiple patients; even if the needle is changed between patients, there can still be body fluid exposure.

Insulin Pens
Insulin is a high-alert medication that can be associated with significant patient harm when used in error. A variety of error types have been associated with insulin therapy, including administration of the wrong insulin product, improper dosing (underdosing and overdosing), dose omissions, incorrect use of insulin delivery devices, wrong route (intramuscular versus subcutaneous), and improper monitoring. Many errors result in serious hypoglycemia or hyperglycemia. Hypoglycemia is often caused by a failure to adjust insulin therapy in response to a reduction in nutritional intake or an excessive insulin dose stemming from a prescribing or dose measurement error. Other factors that contribute to serious hypoglycemia include inappropriate timing of insulin doses with food intake, creatinine clearance, body weight, changes in medications that affect blood glucose levels, poor communication during client transfer to different care teams, and poor coordination of blood glucose testing with insulin administration at meal time.
One strategy for look-alike medications such as Humalog and Humalin is tall man lettering on the label. Tall man lettering describes a method for differentiating the unique letter characters of similar drug names known to be confused with one another, such as HumaLOG and HumaLIN.
ISMP recommends the following safe practice guidelines for the administration of insulin by the nurse:
- Patient-specific insulin pens are stored on clinical units in a manner that prevents their inadvertent use on more than one client.
- A coordinated process is developed to ensure timely blood glucose checks and administration of prandial insulin in conjunction with meal delivery.
- Verbal communication of point-of-care blood glucose value results is avoided as much as possible and is NEVER routinely used as the only source of information when determining insulin doses.
- Appropriately label all clinician-prepared syringes of subcutaneous insulin unless the medication is prepared at the client’s bedside and is immediately administered to the patient without any break in the process.
- Prior to subcutaneous insulin administration, the practitioner:
- Confirms that there is an appropriate indication
- Assesses the patient’s most current blood glucose value
- Assesses the patient for symptoms of hypoglycemia
- Informs the patient of their most current blood glucose level
- Informs the patient of their dose, the full name of the product, and the insulin’s intended action
- An individual insulin pen is never used for more than one client.
- Barcode scanning is used to verify that a patient-specific pen is used to administer the correct insulin to the correct client.
- Prior to transitions of care, a process is in place to ensure that patients will have the necessary prescriptions, supplies, a follow-up care plan, and printed instructions for all prescribed insulin and blood glucose monitoring.
- Patients discharged on insulin are assessed for understanding of their self-management, including:
- Demonstration of proper dose measurement and self-administration using the same administration device that will be used at home (e.g., vial and syringe, pen, pump)
- Correct monitoring of blood glucose values
- The signs and symptoms of hyper- and hypoglycemia and how to respond if these symptoms occur
- Common types of errors possible with their insulin therapy and how to prevent or detect these errors
- The importance of regular follow-up with their primary care provider/specialist, including the date of their next appointment
- Patients who self-administer concentrated U-500 insulin using a vial and syringe are taught to use only a U-500 syringe and communicate their doses in terms of the name and concentration of the insulin and the actual dose in units using only the U-500 syringe.
Lifespan Considerations:
Elderly
The elderly are at higher risk for hypoglycemia episodes. The following are evidence-based recommendations for elderly clients with diabetes:
- In older adults at increased risk of hypoglycemia, medication classes with low risk of hypoglycemia are preferred.
- Overtreatment of diabetes is common in older adults and should be avoided.
- Deintensification (or simplification) of complex regimens is recommended to reduce the risk of hypoglycemia, if it can be achieved within the individualized A1C target.
Children and Adolescents
Type 1 diabetes is the most common form of diabetes in youth. Unique aspects of care and management of children and adolescents with type 1 diabetes must be considered, such as changes in insulin sensitivity related to physical growth and sexual maturation, ability to provide self-care, supervision in the child care and school environment, neurological vulnerability to hypoglycemia and hyperglycemia in young children, as well as possible adverse neurocognitive effects of diabetic ketoacidosis (DKA). Evidence-based recommendations for glycemic control for children and adolescents include:
- The majority of children and adolescents with type 1 diabetes should be treated with intensive insulin regimens, either via multiple daily injections or continuous subcutaneous insulin infusion.
- All children and adolescents with type 1 diabetes should self-monitor glucose levels multiple times daily (up to 6–10 times/day), including pre-meal, pre-bedtime, and as needed for safety in specific situations such as exercise, driving, or the presence of symptoms of hypoglycemia.
- Continuous glucose monitoring should be considered in all children and adolescents with type 1 diabetes, whether using injections or continuous subcutaneous insulin infusion, as an additional tool to help improve glucose control. Benefits of continuous glucose monitoring correlate with adherence to ongoing use of the device.
- Automated insulin delivery systems appear to improve glycemic control and reduce hypoglycemia in children and should be considered in children with type 1 diabetes.
- An A1C target of <7.5% should be considered in children and adolescents with type 1 diabetes but should be individualized based on the needs and situation of the client and family.
There are several different types of insulins that vary in terms of onset, peak, and duration. It is critical for the nurse to be knowledgeable of these differences to help prevent episodes of hypoglycemia due to mismatched administration of insulin with food intake.
Rapid-Acting Insulin
Novolog insulin
Indications: Rapid-acting insulins are also called prandial insulins because they are administered with meals to mimic the effects of endogenous insulin release when food is eaten. Dosages of rapid-acting insulin are individualized based on carbohydrate intake, premeal glucose levels, and anticipated activity.
Mechanism of Action: Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat and by inhibiting hepatic glucose production.
Specific Administration Considerations: Humalog-100 (100 units per ml) and Humalog-200 (200 units per ml) are administered subcutaneously; however, only Humalog-100 is administered via continuous subcutaneous injection or intravenously. Humalog-100 can only be mixed with NPH insulin, but Humalog-200 should not be mixed with other insulin. Inspect insulin visually before use. It should appear clear and colorless; do not use if particulate matter or coloration is seen. Humalog-100 is available in vials, KwikPens, and cartridges; Humalog-200 is only available in KwikPens. Administer subcutaneously into the abdominal area, thigh, or deltoid, and rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy. Lipodystrophy can be a lump or small dent in the skin that forms when a person performs injections repeatedly in the same spot.
Because of the rapid onset of insulin lispro and insulin aspart and the potential for hypoglycemia, these insulins should be administered within 15 minutes before. Peak serum levels are seen 30 to 90 minutes after dosing. Inhaled insulin enters the bloodstream within 1 minute and peaks in 30-60 minutes. Inhaled insulin is contraindicated in clients with chronic lung disease such as asthma or COPD.
Adverse effects of all insulins include hypoglycemia and hypokalemia. Inhaled insulin has a Black Box Warning for potentially causing acute bronchoconstriction.
Patient Teaching & Education: See IMSP guidelines for patient teaching on “High-Risk Medications and Prevention of Errors.” See this link: https://home.ecri.org/blogs/ismp-resources
Short-Acting Insulin
Short-acting insulins include regular insulin, which is marketed under the brand names Humulin R or Novolin R. A concentrated formulation of Humulin R u-500 is also available.
An image of Humulin R insulin.
Humulin R insulin
Indications: Short-acting insulins are given with meals to mimic the effects of endogenous insulin release when food is eaten. Dosages of short-acting insulin are individualized based on carbohydrate intake, premeal glucose levels, and anticipated activity levels.
Mechanism of Action: The primary activity of insulin is the regulation of glucose metabolism. Insulin lowers blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production.
Specific Administration Considerations: Regular insulin is generally administered subcutaneously. It is the only insulin that can be administered intravenously under close supervision of blood glucose and potassium levels. It is available in vials and insulin pens. Inspect insulin visually before use. It should appear clear and colorless; do not use if particulate matter or coloration is seen. Administer subcutaneously into the abdominal area, thigh, or deltoid, and rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy. Subcutaneous doses should be administered approximately 30 minutes before meals because this is the typical onset of action. Peak effects occur in 3 hours with a duration of 8 hours. Do not mix with insulin preparations other than NPH.
Humulin R u-500 should only be administered in u-500 insulin syringes to avoid dosage calculation errors.
Adverse effects of insulin include hypoglycemia and hypokalemia.
Patient Teaching & Education: See IMSP guidelines for patient teaching on “High-Risk Medications and Prevention of Errors.” See this link: https://home.ecri.org/blogs/ismp-resources
Intermediate-Acting Insulin
NPH insulin, also known as isophane insulin, is an intermediate-acting insulin. Brand names include Humulin-N or Novolin-N. Mixtures of short- and intermediate-acting insulin include Humulin 70/30 or Novolin 70/30.
Indications: Intermediate insulins are administered once or twice daily to mimic endogenous basal insulin levels.
Mechanism of Action: Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat and by inhibiting hepatic glucose production.
Specific Administration Considerations: NPH insulin is a white and cloudy suspension. Gently roll or invert the vial/pen several times to re-suspend the insulin before administration. It should only be administered subcutaneously. It may be mixed with rapid-acting or short-acting insulins, but those insulins should be drawn into the syringe before the NPH is added. Administer subcutaneously into the abdominal area, thigh, or deltoid, and rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy. The onset of action and peak is affected by the site of injection, physical activity level, and other variables, but the median peak level occurs in 4 hours. See the image of Novolin-N (cloudy insulin) that can be mixed with Novolin R (clear insulin).
Mixed medications such as Humulin 70/30 should be administered subcutaneously approximately 30 minutes before a meal. They are typically dosed twice daily (with each dose intended to cover 2 meals or a meal and a snack).
Unopened vials should be stored in the refrigerator until the expiration date. Opened vials should be labelled with the open date and stored in the refrigerator for up to 28-42 days (depending on the formulation/insulin type) and then discarded. Unopened pens should be stored in the refrigerator until the expiration date. Used pens should be stored at room temperature, but kept away from heat and light, for up to 10-28 days (depending on the formulation/insulin type) and then discarded.
Patient Teaching & Education: See IMSP guidelines for patient teaching on “High-Risk Medications and Prevention of Errors.” See this link: https://home.ecri.org/blogs/ismp-resources
Examples
Long-Acting Insulin
Insulin glargine (Lantus) and insulin devemir (Levemir) are long-acting insulins given once or twice daily. See the figure below for an image of a Levemir insulin pen.

Indications: Long-acting insulin is given once or twice daily. In type 1 diabetics, it should be used concomitantly with rapid or short-acting insulin at mealtimes.
Mechanism of Action: Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat and by inhibiting hepatic glucose production.
Specific Administration Considerations: Long-acting insulin has a relatively constant concentration/time profile over 24 hours with no pronounced peak compared to NPH insulin. It should only be administered subcutaneously and is available in vials and insulin pens. Inspect insulin visually before use. It should appear clear and colorless; do not use if particulate matter or coloration is seen. Administer subcutaneously into the abdominal area, thigh, or deltoid, and rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy.
Patient Teaching & Education: See IMSP guidelines for patient teaching on “High-Risk Medications and Prevention of Errors.” See this link: https://home.ecri.org/blogs/ismp-resources
Patient Teaching & Education:
Insulins Medication Card

Diabetic Medication: Oral Antihyperglycemics
There are several different classes of oral antihyperglycemic drugs used in conjunction with a healthy diet and exercise for the management of type 2 diabetes. Currently, metformin is the preferred initial pharmacologic agent for the treatment of type 2 diabetes. Three of the most commonly used antihyperglycemic classes and prototypes are sulfonylureas (glipizide), biguanide (metformin), and DPP-IV (sitagliptin). The mechanism of action and administration considerations for each of these prototypes are described below.
Therapeutic Effects: management of type 2 diabetes
| Class | Prototypes | Administration Considerations | Therapeutic Effects | Adverse/Side Effects |
| Sulfonylureas | Glipizide | Time with meals; peak plasma concentrations occur 1 to 3 hours after administration | Reduce fasting blood sugar and glycosylated hemoglobin to near normal | Hypoglycemia; may be potentiated by nonsteroidal anti-inflammatory agents and other drugs that are highly protein bound |
|---|---|---|---|---|
| Biguanide | Metformin | Contraindicated in renal and hepatic disease
Should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials |
Reduce fasting blood sugar and glycosylated hemoglobin to near normal | Stop immediately if signs of lactic acidosis or any condition associated with hypoxemia, dehydration, or sepsis occurs
Common adverse effects: diarrhea, nausea/vomiting, weakness, flatulence, indigestion, abdominal discomfort, and headache |
| DPP-IV inhibitor | Sitagliptin | Can be given with or without food | Reduce fasting blood sugar and glycosylated hemoglobin to near norm | Hypoglycemia
Report hypersensitivity reactions, blisters/erosions, headache, or symptoms of pancreatitis, heart failure, severe arthralgia, or upper respiratory infection |
Summary of Diabetes Care
The example concept map below provides a summary of the key information necessary to understand glucose regulation. The concept map below demonstrates how the pathology of diabetes dictates the patient’s plan of care.

Glucose Regulation Concept Map
A summary case study:
A patient with diabetes mellitus type 2 is admitted to the hospital for hip replacement surgery. The nurse reviews the following orders:
Bedside blood glucose testing before meals (q ac) and at bedtime (q hs) with sliding scale Humalog insulin
Sliding scale Humalog insulin based on pre-prandial glucose level:
- 60-140 mg/dl: No coverage
- 141-160 mg/dl: 2 units
- 161-180 mg/dl 4 units
- 181-200 mg/dl 6 units
- 201 – 220 mg/dl 8 units
- Over 220 mg/dl: call the provider
Metformin 1000 mg twice daily
Humulin-N 20 units at breakfast and at bedtime
Hypoglycemia protocol will be initiated if the glucose level is less than 50 mg/dl
Answer the following questions based on the information provided by the case scenario:
- Explain the difference between type 1 and type 2 diabetes.
- The patient states that he usually does not take insulin at home. What is the likely rationale for insulin therapy while hospitalized?
- The patient’s blood sugar before breakfast is 180 mg/dl. What types and amounts of insulin will the nurse administer?
- The nurse reviews the patient’s morning lab results and finds a blood creatinine of 1.3 mg/dl. She plans to call the provider to discuss the impact of the results on the medications ordered. Which medication may require a dosage adjustment based on these results?
- When the nurse enters the room around 4 p.m., she discovers that the patient has become irritable and is shaky. The nurse performs a bedside blood glucose and obtains a value of 44 mg/dl. What is the nurse’s best response?
- What is the likely cause of the patient’s condition? Explain using the onset and peak actions of the insulin orders.
- On admission, the patient’s A1C level was 10%. What does this lab value indicate?
- The provider states the discharge plan is to initiate Lantus insulin therapy at home based on the admitting A1C level. What patient teaching should the nurse plan to provide before discharge?
Note: Answers to this case study will be discussed in class.

