2 Clinical Judgement: Foundations of Pharmacology and Pathophysiology
This chapter will introduce clinical judgment, how it relates to the study of drugs and the foundational concepts of pharmacology and pathophysiology in nursing. Clinical judgment is at the heart of nursing practice, guiding nurses in making informed decisions that ensure patient safety and optimal outcomes. Clinical judgment is deeply rooted in a thorough understanding of foundational concepts such as pathophysiology and pharmacology. Pathophysiology provides nurses with the knowledge of how diseases affect the body, enabling them to recognize signs and symptoms, anticipate complications, and understand the rationale behind clinical interventions. Pharmacology, on the other hand, equips nurses with the essential knowledge of how drugs interact with the body, including their therapeutic effects, potential side effects, and mechanisms of action. The integration of clinical judgment with these foundational concepts is crucial for safe and effective medication administration. A nurse’s ability to critically analyze a patient’s condition, understand the pharmacokinetics and pharmacodynamics of prescribed medications, and anticipate potential drug interactions or adverse effects directly influences the quality of care provided. This chapter will explore the essential elements of pharmacology that underpin clinical decision-making in nursing practice, leading to the following learning objectives:
Learning Objectives
- Describe drug generic names, trade names, and prototypes.
- Discuss major drug laws, standards, regulations, and safety drug administration.
- Discuss the mechanism of action in relation to drug therapy.
- Describe the process of pharmacokinetics, including drug absorption, distribution, metabolism, and excretion.
- Identify drug-related and patient-related variables that affect drug actions and potential effects of drug-drug interactions.
- Demonstrate knowledge needed to administer medication to a patient, including signs and symptoms of adverse drug effects and rights of medication administration.
- Apply steps of the nursing process and evidence-based practice research in medication administration.
- Discuss medication errors based on their incidence, cause, and prevention.
I: Chapter Overview of Clinical Judgement Connection
Clinical judgment is the accumulation of knowledge and skill over time that contributes to the nurse’s ability to analyze and synthesize the patient’s presentation. Objective and subjective data guide the nurse’s clinical decision-making and provide evidence-based nursing interventions to help improve patient outcomes. This chapter will also introduce the clinical judgment management model (CJMM), the nursing process, and how it relates to safety and quality with medication administration, pharmacokinetics, and pharmacodynamics.
What is the Connection with Clinical Judgement and Pharmacology
- Assessments that are holistic
- Critical thinking
- Clinical reasoning
- Hands-on practice
- Intuition
- Reflection
The nursing profession contains multiple daily tasks that a practicing nurse must complete. One of the top priorities is medication administration. Medication
II. Pharmacokinetics
Pharmacokinetics is drug movement through the body to achieve its required action. This phase involves four essential processes: absorption, distribution, metabolism, and excretion.
1. Absorption: Medications come in various forms. The first stage of pharmacokinetics is known as absorption. Absorption occurs after drugs enter the body and travel from the administration site into circulation. Medications can enter the body through various routes of administration. Standard routes to administer medications include the following examples:
-
- oral (swallowing a tablet)
- enteral (administration through the gastrointestinal tract, such as through a tube)
- rectal (administration of a suppository)
- inhalation (breathing in medication from an inhaler)
- intramuscular (getting an injection into a muscle)
- subcutaneous (an injection into the fat tissue beneath the skin)
- transdermal (placement of a patch on the skin)
- intravenous (injection directly into a vein)
When a medication is administered orally or enterally, it faces its biggest hurdle during absorption in the gastrointestinal (GI) tract. Medications made of protein that are swallowed or otherwise absorbed in the GI tract may quickly be deactivated by enzymes as they pass through the stomach and duodenum. Suppose the drug does get into the blood from the intestines. In that case, part of it will be broken down by liver enzymes, known as the first-pass effect, and some of it will escape to the general circulation to either become protein-bound (inactive) or stay free (and create an action at a receptor site). When it comes to absorption, oral medications will significantly be affected by the first-pass effect, and this can affect the percentage of the administered drug that will be available to complete the required action (this is known as bioavailability). The bioavailability of medications administered through the oral route is always less than 100% and will vary based on the rate of first-pass metabolism.
A workaround to the first-pass effect is to administer the medication using alternate routes such as dermal, nasal, inhalation, injection, or intravenous. Alternative routes of medication administration bypass the first-pass effect by entering the bloodstream directly or via absorption through the skin or lungs. Medications administered directly into the bloodstream (referred to as intravenous medications) do not undergo absorption and are fully available for distribution to tissues within the body.
2. Distribution: The second step of pharmacokinetics involves drug distribution. Distribution is the process by which medication is dispersed throughout the body via the bloodstream. Once a drug enters systemic circulation by absorption or direct administration, it must be distributed into interstitial and intracellular fluids to reach the target cells. A drug’s distribution throughout the body depends on common factors such as blood flow, plasma protein binding, lipid solubility, blood-brain, and placental barriers. Other factors include capillary permeability, differences between blood/tissue, and volume of distribution.
Distribution of medication can also cause unintended adverse effects or side effects. Drugs are designed to primarily cause one effect, meaning they bind more strongly to one specific receptor site and predictably cause or block an action. However, side effects can occur when the drug binds to other sites in addition to the target tissue, causing secondary side effects. These side effects can range from tolerable to unacceptable, resulting in the medication’s discontinuation. For example, a person might take the pain reliever ibuprofen (Advil) to treat a sore leg muscle, and the pain may be subsequently relieved, but there may also be stomach irritation as a side effect that may cause the person to stop taking Ibuprofen.
3. Metabolism: The third step is drug metabolism (biotransformation). In this phase, drugs are chemically altered by the body into a form that allows them to be excreted from the body. The breakdown of a drug molecule usually involves two steps, primarily in the body’s chemical processing plant: the liver. Everything that enters the bloodstream—whether swallowed, injected, inhaled, absorbed through the skin, or produced by the body itself—is carried to this largest internal organ.
4. Excretion: The final process in pharmacokinetics is excretion. The body has absorbed, distributed, and metabolized the medication molecules and will eliminate it from the body. The kidney often filters the remaining parent drugs and metabolites in the bloodstream, where a portion undergoes reabsorption back into the bloodstream, and the remainder is excreted in the urine. The liver also excretes byproducts and waste into the bile. Another potential route of excretion is the lungs.
Kidneys
The most common route of excretion is the kidney. As the kidneys filter blood, most drug byproducts and waste are excreted in the urine. The excretion rate can be estimated by considering several factors: age, weight, biological sex, and kidney function. Kidney function is measured by lab values such as serum creatinine, glomerular filtration rate (GFR), and creatinine clearance. If a patient’s kidney function is decreased, then their ability to excrete medication is affected, and drug dosages must be altered for safe administration.
Liver
As the liver filters blood, some drugs and their metabolites are transported by the hepatocytes (liver cells) to bile. Bile moves through the bile ducts to the gallbladder and then onto the small intestine. During this process, some drugs may be partially absorbed by the intestine back into the bloodstream. Other drugs are biotransformed (metabolized) by intestinal bacteria and reabsorbed. Unabsorbed drugs and byproducts/metabolites are excreted via the feces. If a patient is experiencing decreased liver function, their ability to excrete medication is affected, and drug dosages must be reduced. Lab studies used to estimate liver function are called liver function tests and include measurement of the Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) enzymes that the body releases in response to damage or disease.
Other Routes to Consider
Sweat, tears, reproductive fluids (such as seminal fluid), and breast milk can also contain drugs and byproducts/metabolites of drugs.
III. Pharmacodynamics
In the pharmacodynamic phases, chemical reactions occur that produce the drugs’ effect on the body and the mechanism of action. When considering how the cells of the body respond to medications, it is important to remember that the majority of drugs bind to specific receptors on the surface or interior of cells. However, many other cellular components and non-specific sites can serve as receptor sites where drugs can bind to create a response. Drug responses can be desirable and undesirable. For example, if a patient is administered diphenhydramine (Benadryl) for itching related to contact dermatitis (triggered by an allergic reaction), the desired effect will be that the itching stops, but the undesired effect will be drowsiness and/or sedation that will result from depression of the central nervous system (CNS).
In pharmacodynamics, the main goal of drug administration is for the drug to reach a therapeutic range in the body wherein it can achieve the desired effect. However, there are some drugs that have a narrow therapeutic index that, once they enter the circulatory system toxicity, can result in an adverse (undesirable) effect that is unsafe and/or harmful for a patient. The blood concentration can become toxic; therefore, some drugs must be continuously monitored once a steady state (amount of drug administered equals the amount being eliminated) has been achieved. These concentrations are known as the peak (the highest concentration drawn at a minimum of one hour to several hours after the drug is administered) and trough (the lowest concentration drawn immediately before the next scheduled dose of a drug).
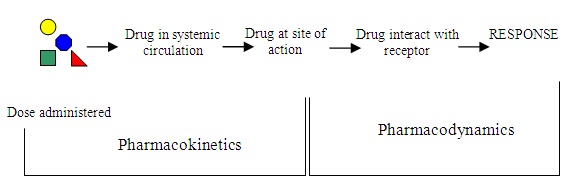
Understanding the mechanism of action, or how a medication functions within the body, is essential to understanding the processes medications go through to produce the desired effect. Drugs have agonistic or antagonistic effects. A drug agonist binds tightly to a receptor to produce the desired effect. A drug antagonist competes with other molecules and blocks a specific action or response at a receptor site. The process of pharmacodynamics has other important aspects that should be considered. These aspects include the onset of action, the peak time, and the duration of action once a drug has been administered. The picture below helps you to see the role of pharmacokinetics and pharmacodynamics:
IV. Pathophysiology and Medication Administration
Pathophysiology and pharmacology are interrelated and play a key role in nursing practice. While pathophysiology explains a disease process’s functional and chemical properties, pharmacology connects drugs’ effects on the body. The knowledge that you will gain from pathophysiology and pharmacology will enable you to understand how your patient’s treatment works, their disease process, side effects, and how to safely and effectively monitor and manage their condition. Knowledge of pathophysiology and pharmacology is also needed for you to educate your patient about their condition and treatment in a simple way that they can understand. You will also be in a position to discuss your patient effectively with members of a multi-professional team. It is important to understand that knowing the normal function of body systems (respiratory, cardiovascular, gastrointestinal, central nervous system, endocrine, etc.) can greatly help you promptly identify and understand when an organ system fails or is functioning abnormally.
A disease process can affect a patient’s clinical presentation. Patients can present in a healthcare facility with a variety of clinical signs and symptoms, and in order for you to get a full picture of a disease or condition, you must understand which body system is affected, the clinical manifestations that your patient will present with and how a particular drug will work to fix the dysfunction. Part of your role as a nurse will be to deliver person-centered care that is holistic and individualized. Applying pathophysiology and pharmacology will assist you with your clinical decision-making, strengthen your assessment of your patients, and help you use a safe and effective systematic process that is evidence-based to properly plan your care and interventions and to accurately evaluate your patient care outcomes. In this book, you will learn conceptually about particular organ systems, disease processes, and the classifications of medications that are used for management.
V. Improving Medication Safety for Nurses
- Establish safe work environments for medication preparation, administration, and documentation. Reduce distractions and provide appropriate lighting, for instance.
- Maintain a culture of rigorous commitment to principles of safety in medication administration (for instance, the five rights of medication safety and cross-checks with colleagues, where appropriate).
- Remove barriers and facilitate the involvement of patient surrogates in checking the administration and monitoring the medication effects.
- Foster a commitment to patients’ rights as co-consumers of their care.
- Develop aids for patients or their surrogates to support self-management of medications.
- Enhance communication skills and team training to be prepared and confident in questioning medication orders and evaluating patient responses to drugs.
- Actively advocate for the development, testing, and safe implementation of electronic health records.
- Work to improve systems that address “near misses” in the work environment.
- Realize they are part of a system and do their part to evaluate the efficacy of new safety systems and technology.
- Contribute to the development and implementation of error reporting systems and support a culture that values accurate medication error reporting.
Key Steps for Ensuring Medication Safety:
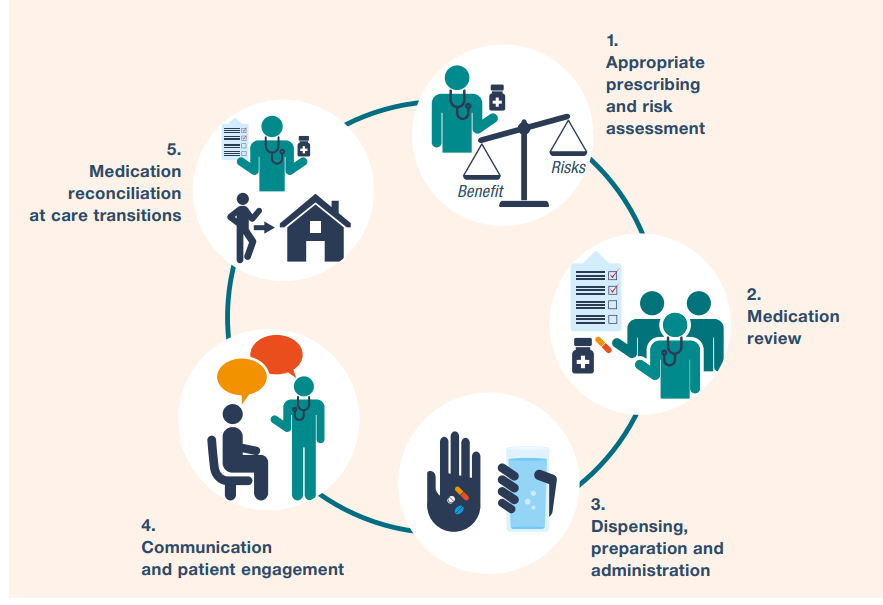
Medication safety in high-risk situations includes high-risk
medications, provider-patient relations, and systems factors.
Medication administration is an essential task nurses perform while providing patient care. However, safe medication administration is more than just a nursing task; it is a process involving several members of the health care team, as well as legal, ethical, social, and cultural issues. The primary focus of effective medication administration by all health professionals is patient safety. Although many measures have been put into place over the past few decades to promote improved patient safety, medication errors, and adverse effects continue to be a common event.
VI. Legal and Ethical Regulations
There are many federal and state laws, as well as national guidelines, that have been established to protect public health and safety. This section will explain how the Food and Drug Administration (FDA), Drug Enforcement Administration (DEA), Joint Commission, Centers for Medicare and Medicaid Services (CMS), a State’s Nurse Practice Act, State Boards of Nursing, and state legislatures protect the consumer from medication harm. To protect the public the U.S. Food and Drug Administration (FDA) is responsible for protecting public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices and by protecting our nation’s food supply, cosmetics, and products that emit radiation.[1] The FDA protects public health regarding medications by enforcing an official drug approval process based on evidence-based research, issuing Black Box Warnings for medications with severe adverse reactions, and regulating over-the-counter (OTC) medications.
Developing New Drugs
American consumers benefit from having access to the safest and most advanced pharmaceutical system in the world. The leading consumer watchdog in this system is the FDA’s Center for Drug Evaluation and Research (CDER).
The center’s best-known job is evaluating new drugs before they can be sold. CDER’s evaluation not only prevents quackery but also provides doctors and patients with the information they need to use medicines wisely. The center ensures that drugs, both brand-name and generic, work correctly and that their health benefits outweigh their known risks.
Development and Approval Process of Drugs by the FDA
Drug companies conduct extensive research and work to develop and test a drug. The company then sends CDER the evidence from these tests to prove the drug is safe and effective for its intended use. Before the drug is approved as safe for use in the United States, a team of CDER physicians, statisticians, chemists, pharmacologists, and other scientists reviews the company’s data and proposed labeling. If this independent and unbiased review establishes a drug’s health benefits outweigh its known risks, the drug is approved for sale. Before a drug can be tested in people, the drug company or sponsor performs laboratory and animal tests to discover how the drug works and whether it’s likely to be safe and work well in humans. Next, a series of clinical trials involving volunteers is conducted to determine whether the drug is safe to treat a disease and whether it provides a real health benefit.
FDA Approval: What it Means
FDA approval of a drug means that CDER has reviewed data on its effects, and the drug is determined to provide benefits that outweigh its known and potential risks for the intended population. Although many of the FDA’s risk-benefit assessments and decisions are straightforward, sometimes the benefits and risks are uncertain and complex to interpret or predict. The agency and the drug maker may reach different conclusions after analyzing the same data, or there may be differences of opinion among members of the FDA’s review team. As a science-led organization, the FDA uses scientific and technological information to make decisions through a deliberative process.
Black Box Warnings
The Food and Drug Administration (FDA) approves a drug for marketing after determining that the drug’s benefits of use outweigh the risks for the condition that the drug will treat. However, even with the rigorous FDA evaluation process, some safety problems surface only after a drug has been on the market and used in a broader population. If a safety problem surfaces, the FDA issues Black Box Warnings that appear on a prescription drug’s label. The purpose is to call attention to serious or life-threatening risks.
U.S. Drug Enforcement Agency (DEA)
The U.S. Drug Enforcement Agency (DEA) enforces the laws and regulations for controlled substances in the United States. This includes enforcement of the Controlled Substances Act (CSA) that pertains to the manufacture, distribution, and dispensing of legally produced controlled substances that nurses administer to patients. Because controlled substances have a greater chance of being misused and abused, some additional laws and procedures must be followed when working with these medications. The federal government administers some laws regarding controlled substances. The DEA is responsible for enforcing these laws, and many federal laws are summarized in a document called the Pharmacist’s Manual. Most controlled substance laws, however, come from the state governments. Healthcare professionals are responsible for following the most stringent of the two laws, whether state or federal.
Federal Laws
The following are excerpts of federal laws that apply to professional nursing.
Prescriptions: A prescription for a controlled substance may be written only by a provider (physician or mid-level provider like a nurse practitioner) that has a DEA registration number. A prescription for a Schedule II (the most controlled class of medications, like opioids) must be written or electronically sent to the pharmacy through DEA-approved software. Prescriptions over the phone or fax are not accepted. It is then up to state law to decide how long a written Schedule II prescription is valid and if there are any limits on the quantity of medication that can be dispensed. Refilling a Schedule II medication is not allowed. Schedule III or IV medications may be refilled only five times.
Generic Medications: Generic medications can be safe and effective alternatives to their brand-name counterparts, often at a reduced cost. By law, generic medications must have the same chemically active ingredient in the same dose (i.e., they must be “bio-equivalent”). However, the excipients (the base substance that holds the active chemical ingredient into a pill form (such as talc) or the flavoring can be different. Some patients do not tolerate these differences in excipients very well. The provider must indicate that generic substitution is acceptable when prescribing a medication.
Over-the-counter Medications: Over-the-counter (OTC) medications do not require a prescription. They can be bought at a store and used by multiple individuals. OTC medications are also regulated. Some prescription medications, such as OTC, are available for purchase in smaller doses. For example, diphenhydramine (Benadryl) is commonly prescribed as 50 mg every 6 hours, and the prescription strength is 50 mg. However, OTC can also be purchased in 25 mg doses (or less for children.)
Herbals & Supplements: Herbs and supplements may include various substances such as vitamins, minerals, enzymes, and botanicals. Supplements such as “protein powders” are marketed to build muscle mass and can contain different substances that may not be appropriate for all individuals. The FDA is responsible for authorizing natural/non-prescription products for which safety, efficacy, and quality standards are in place. Some herbal and supplement substances are not regulated, and most have not undergone rigorous scientific testing for public safety. While individuals may be tempted to try these herbals and supplements, there is no guarantee that they contain the ingredients listed on the label. It is also important to remember that there is a potential for adverse effects or even overdose if the herbal or supplement contains some of the same drug that was also prescribed to a patient.
Records: There is a “closed system” for keeping records of controlled substances to prevent diversion. To maintain a “closed system” of record keeping for controlled substances, hospitals, clinics, and pharmacies must maintain records on the whereabouts of the medication from manufacturing the medication, receipt by the pharmacy, distribution to the patient, and disposal of waste. What does this look like in practice? Inventory counts of controlled medications occur frequently, controlled substance access by individual employees is audited often, detailed records are kept for all transactions, and waste is usually disposed of differently than other pharmaceuticals.
Drug overdoses are still a public health crisis in the United States, and the misuse of prescription opioids, which are scheduled medications, continues to contribute to a large percentage of overdose deaths. Many problems associated with drug abuse are the result of legitimately made controlled substances being diverted from their lawful purpose into illicit drug traffic. The mission of DEA’s Diversion Control Division is to prevent, detect, and investigate the diversion of controlled pharmaceuticals from legitimate sources while ensuring an adequate and uninterrupted supply for legitimate medical, commercial, and scientific needs. The DEA provides education regarding related topics that apply to nurses, such as drug diversion, state prescription drug monitoring systems, current drug trends, telemedicine, and proper drug disposal.
Drug Diversion: Drug diversion involves the transfer of any legally prescribed controlled substance from the individual for whom it was prescribed to another person for any illicit use. The most common drugs diverted from the health care facility setting are opioids. Diversion of controlled substances is not uncommon and can result in substantial risk not only to the individual who is diverting the drugs but also to patients, coworkers, and employers. Impaired providers can harm patients by providing substandard care, denying medications to patients, or exposing patients to tainted substances. Tampering is the riskiest and most harmful type of diversion. Commonly, the diverter removes medication from a syringe, vial, or other container and injects himself or herself with the medication. The diverter then replaces the stolen medication with saline, sterile water, or another clear medication or liquid. An unaware provider later uses the “replacement liquid” on the patient. When tampering, the diverter may rarely use a sterile technique. Ultimately, the patient doesn’t receive the required medication and may be exposed to the diverter’s blood.
CMS: Centers for Medicare and Medicaid Services
The Centers for Medicare & Medicaid Services (CMS) is a federal agency within the United States Department of Health and Human Services (HHS) that administers the Medicare program and works with state governments to administer Medicaid. The CMS establishes and enforces regulations to protect patient safety in hospitals that receive Medicare and Medicaid funding. CMS regulations related to medication administration include identifying what should be included in a prescription, using the “five rights” when administering medications, reporting concerns about a medication order, assessing and monitoring patients receiving medications, and documenting medication administration. Each of these regulations is further discussed below.
Medication Orders
Medications must be administered in response to an order from a practitioner or based on a standing order that is appropriately authenticated subsequently by a practitioner. All practitioner orders for the administration of drugs and biologicals must include at least the following:
- Name of the patient
- The patient’s age and weight facilitate dose calculation when applicable. Policies and procedures must address weight-based dosing for pediatric patients and other circumstances identified in the hospital’s policies. (Note that dose calculations are based on metric weight (kg or g for newborns)
- Date and time of the order
- Drug name
- Dose, frequency, and route
- Dose calculation requirements, when applicable
- Exact strength or concentration, when applicable
- Quantity and/or duration, when applicable
- Specific instructions for use, when applicable
- Name of the prescriber
Safe Practices For Medication Administration: The Five Basic Rights
CMS states that hospitals’ policies and procedures must reflect accepted standards of practice that require the following information is confirmed prior to each administration of medication. This is often called the “five rights” of medication administration practice.
Note: Recent literature has identified up to nine “rights” of medication administration, including Right patient, Right drug, Right route, Right time, Right dose, Right documentation, Right action (appropriate reason), Right form, and Right response. However, there does not (yet) appear to be a consensus about expanding beyond the 5 “rights.”
Many agencies have implemented barcode medication scanning to improve safety during medication administration. Bar code scanning systems reduce medication errors by electronically verifying the “5 rights” of medication administration. For example, when a nurse scans a bar code on the patient’s wristband and on the medication to be administered, the data is delivered to a computer software system where algorithms check various databases and generate real-time warnings or approvals. Research studies have shown that bar code scanning reduces errors resulting from the administration of a wrong dose or wrong medication, as well as errors involving medication being given by the wrong route. However, it is important to remember that bar code scanning should be used in addition to performing the five rights of medication administration, not in place of this important safety process. Additionally, nurses should carefully consider their actions when errors occur during the bar code scanning process. Although it may be tempting to quickly dismiss the error and attribute it to a technology glitch, the error may have been triggered due to a patient safety concern that requires further follow-up before the medication is administered. It is important for nurses to investigate errors that occur during the bar code scanning process just as they would do if an error is discovered during the traditional five rights of medication process.
Putting It All Together in a Video Case Scenario:
Case Study #1: Medical Errors
As you watch this video, think of and write down your answer(s) to the following questions:
- What are the contributing factors that led to Mr. Water’s condition? Was this situation preventable? Why or why not?
- The patient is currently taking medications for his blood pressure (Norvasc); cholesterol (Lipitor); and a blood thinner (Coumadin). With the knowledge you have gained about medication safety, what nursing interventions would you have done differently to maintain the patient’s safety? Apply the components of the fundamental rights of medication administration.
- Briefly include information about communication in the video. Is this a necessary process? Why or why not? Identify any breaks that interfered with safety that led to Mr. Water’s condition.
Putting It All Together in a Video Case Scenario:
Case Study#2: Administering Cardiac Medications to A Patient with Dementia
In this case study, you will have a patient who has dementia. Dementia is a group of diseases that affects an individual’s cognitive function. It can affect their ability to think, remember, and make good decisions, hindering their ability to complete their daily activities. In the first case study, the patient was cognitively intact, but in this case study, you will face the challenge of caring for a patient with cognitive impairment. Your objectives in this case study are to:
- Maintain a safe, effective care environment for adults of all ages
- Use appropriate communication techniques
- Use the nursing process to help guide your clinical judgment
- Adapt nursing practice to meet the needs of diverse patients in a variety of settings
How will you change your approach to meet the needs of this patient? Remember that safety is ALWAYS A NUMBER ONE PRIORITY regarding medication administration. Click on the link to access the interactive virtual simulation.
Media Attributions
- liver (front) with gallbladder © unknown is licensed under a CC BY (Attribution) license
- Fármacos_farmacocinética_e_farmacodinâmica
- Medication Safety in High Risk Situations_WHO © World Health Organization is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license

